There is a battle taking place across the globe between civil liberties, medical tyranny and government overreach. The issue of mandatory COVID-19 investigational vaccinations has been at the forefront of this contentious battle globally and among citizens of South Carolina. On Thursday September 9, 2021, Biden issued a statement that all Federal employees and businesses with greater than 100 employees must make the COVID-19 biologic inoculation to maintain employment. This comes on the heels of both the City of North Charleston and the City of Charleston imposing the same mandatory inoculations.
First, it is critical that we understand the Food and Drug Administration (FDA) documents released regarding “full approval.” According to the FDA document issued on August 23, 2021, stating that the Emergency Use Authorization (EUA) was expanded for the Pfizer-BioNTech COVID‑19 “investigational” biologic to the age groups 16 years of age and older. In this document, it also states that there was a need “to update language regarding warnings and precautions related to myocarditis and pericarditis.”
The FDA letter regarding COMINARTY and BioNTech states that they can be used interchangeably:
COMIRNATY (COVID-19 Vaccine, mRNA) is the same formulation as the Pfizer-BioNTech COVID-19 Vaccine and can be used interchangeably with the Pfizer-BioNTech COVID-19 Vaccine to provide the COVID-19 vaccination series.
The licensed vaccine has the same formulation as the EUA-authorized vaccine and the products can be used interchangeably to provide the vaccination series without presenting any safety or effectiveness concerns. The products are legally distinct with certain differences that do not impact safety or effectiveness.
COMINARTY FACT SHEET:
“This EUA for the Pfizer-BioNTech COVID-19 Vaccine and COMINARTY will end when the Secretary of HHS determines that the circumstances justifying the EUA no longer exist or when there is a change in the approval status of the product such that an EUA is no longer needed.”
What does this mean? The full approval granted to the COMINARTY Vaccine is not as it appears. According to the fact sheet for COMINARTY, it is still under the EUA. By doing this, the FDA just freed Pfizer from all liability for either product name, COMINARTY or the BioNTech Vaccines. The only vaccine version in circulation is the Pfizer BioNTech, which is not the legally named approved version. Pfizer BioNTech must still go through evaluation with vaccine studies through 2023, and the final reporting is due in 2027. The public has been led to believe through government FDA sleight of hand, press announcements and Joe Biden speech that the two vaccines, by two different manufacturers, in two different countries, are the same so just take it with no disclosure of ingredients and prior to clinical trial completion. This is strangely reminiscent to the roll out of the 1976 Swine Flu Vaccine, which had devastating consequences.
Author, economic researcher, and historian F. William Engdahl said:
It seems the FDA executed a clever ruse in which it issued separate rulings for a Pfizer Inc.-BioNTech vaccine which is widely used in the USA, and another ruling for the similar vaccine of Pfizer’s German-based partner and developer of the mRNA platform, BioNTech of Mainz. It is only BioNTech that got FDA approval, but conditioned on completion of a series of further tests on select groups including infants, pregnant women and youth, by 2027. The US vaccine, Pfizer-BioNTech Covid-19 vaccine, only got extension of its Emergency Use Authorization (EUA), not full approval!
The public has been led to believe through government FDA sleight of hand, press announcements and Joe Biden speech that it is the same so just take it with no disclosure of ingredients and prior to clinical trial completion.

Under the EUA, the FDA waives current good manufacturing practices (CGMP) requirements. Since both product names are under the EUA and most have not done their homework, an individual cannot be forced to take these inoculations. Per the EUA language, products are not approved and may or may not be effective:
EUA candidate products include medical products and uses that are not approved, cleared, or licensed under sections 505, 510(k), and 515 of the FD&C Act or section 351 of the PHS Act.
Medical products that may be considered for an EUA are those that “may be effective” to prevent, diagnose, or treat serious or life-threatening diseases or conditions that can be caused by a CBRN agent(s) identified in the HHS Secretary’s declaration of emergency or threat of emergency under section 564(b). Potential EUA products also include those that may be effective to mitigate a disease or condition caused by an FDA-regulated product (including a product authorized for emergency use under section 564 or an approved product) used to diagnose, treat, or prevent a disease or condition caused by a CBRN agent.
The “may be effective” standard for EUAs provides for a lower level of evidence than the “effectiveness” standard that FDA uses for product approvals.21 FDA intends to assess the potential effectiveness of a possible EUA product on a case-by-case basis using a risk-benefit analysis, as explained below. If, based on the totality of the scientific evidence available, it is reasonable to believe that the product may be effective for the specified use, FDA may authorize its emergency use, provided that other statutory criteria for issuing an EUA also are met.
Israel has turned into a pharmaceutical #BananaRepublic, where the priorities of a multinational supercedes the priorities of its citizens. It is no longer the Jewish motherland, it is #Pfizerland.
Israel's national anthem should include the words:
"Pfizer Pfizer Uber Alles!"
— Ehden @ TELEGRAM CHANNEL: T.ME/EH_DEN (@eh_den) July 13, 2021
If any of the above is alarming to you, you may ask, “How are the vaccine companies exempt from liability?” Ultimately, it is the contract between countries and Pfizer. America’s Frontline Doctors just released the contents of a contract between Pfizer and Brazil stating that Pfizer is not liable for any damages because of the vaccine and, even if there are any viable treatments for COVID-19, countries cannot void the contract. The contract also states that Pfizer will stipulate the number of doses to be given to individuals, not any other entity, and that they are “exempt” from laws.
“If you were wondering why Ivermectin was suppressed, it is because the agreement that countries had with Pfizer does not allow them to escape their contract, which states that even if a drug will be found to treat COVID-19, the contract cannot be voided.” — America’s Frontline Doctors
Couple the above with the nature of the pharmaceutical contracts, the Public Readiness Emergency Preparedness (PREP) Act signed into act by President Bush in 2005 (*later amended by President Obama and Administrator Biden), led to the vaccine manufacturer and “licensed health professional” immunity from liability under the EUA to include for COVID-19 Vaccinations as well as waived formal consent for citizens. In addition to the sleight of hand by the FDA, CDC and the Federal Government, the military also stated in their letter to all military personnel that a person with a prior infection of COVID-19 is not considered to have immunity and must still undergo involuntary investigative inoculation.
According to numerous testimonies, research, and interviews, Dr. Peter McCullough, Dr. Jane Ruby, Dr. Judy Mikovitz and many others indicate that natural immunity is far more potent and is safer than the vaccines. In fact, a study out of Israel found that those with natural immunity are 1,300% better protected against COVID-19. Regarding the natural immunity and military vaccine mandates, a class action suit was filed by two staff sergeants on August 17, 2021 against the Department of Defense, Department of Health and Human Services and the FDA states that:
Army Regulation 40-562 (“AR 40-562”) provides documented survivors of an infection, a presumptive medical exemption from vaccination because of the natural immunity acquired as a result of having survived the infection. “General examples of medical exemptions include the following… Evidence of immunity based on serologic tests, documented infection, or similar circumstances.” AR 40-562, ¶2-6a.(1)(b).
Plaintiffs also seek a declaratory judgment on the separate basis that the Emergency Use Authorization (“EUA”) DoD COVID-19 Vaccine mandate, which they have been notified is imminent, cannot be issued in violation of 10 U.S.C. §1107 and its implementing regulations, including DoD Directive 6200.2, the FDA regulation of biologics at 21 C.F.R. § 50 et seq., as well as the law regarding informed consent 50 U.S.C. 1520 (“The Nuremburg Code”).
Based on the Nuremberg Code, an individual must provide voluntary (not coerced) informed consent. Further, the investigational product must provide “fruitful results.” To-date the inoculation neither prevents the transmission or catching COVID-19 and its mutations. The investigational product must also avoid all unnecessary physical and mental suffering.
As of September 9, 2021, the COVID Vaccines resulted in:
- 14,506 Covid vaccine Reported deaths
- 60,741 hospitalizations
- 77,919 urgent care visits
- 7,911 cases of Bell’s Palsy
- 1,757 miscarriages
- 6,422 heart attacks
- 5,371 myocarditis and pericarditis cases
- 18,439 permanently disabled individuals
- 701,559 Covid Vaccine Adverse Event Reports
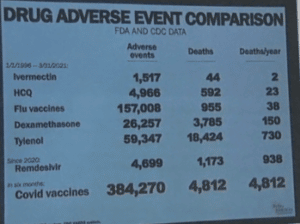
Adding to the events due to these deadly investigational biologics, the inoculated individuals are leading to mutations through Viral Immune Escape and Antibody Dependent Enhancement (ADE).
How are the pharmaceutical companies getting away with this? Simple, the EUA and some good old fashioned manipulation. In order for the Pfizer, Moderna, AstraZeneca and Johnson and Johnson inoculations to be classified under EUA, there must exist no other treatment for the virus. Therefore, there is a constant attack on prophylaxis and COVID-19 treatment with Hydroxychloroquine (HCQ) and Ivermectin.
For FDA to issue an EUA, there must be no adequate, approved, and available alternative to the candidate product for diagnosing, preventing, or treating the disease or condition.
Hydroxychloroquine (HCQ) and Ivermectin have been shown in studies and in use to be highly effective in preventive, early treatment, and treatment in general for the virus as well as other viruses. Both drugs have been on the WHO approval list for several decades. Over 63 trials and 31 randomized control trials show the clear benefits of Ivermectin, which was approved by the FDA in 1996 and won the Nobel prize in 2015. Over 200 studies show the efficacy of HCQ, which was approved in 1955. Within 3 days of Joe Rogan taking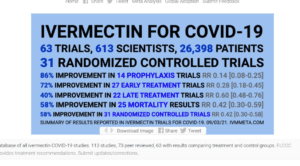
In a blatant attempt to discredit ivermectin, one physician stated that people were overdosing on ivermectin was debunked by the hospital system. The hospital system stated the physician, although he was in the physician group that services the hospitals, had not been working in their hospitals for some time. Attempts to stop the use of ivermectin has trickled down to pharmacies across the US.
Pharmacies are now denying ivermectin scripts to patients, such as Delta Pharmacy in Charleston, SC who denied us a statement. If the pharmacy fills the script, such as Publix, insurance has now been instructed not to cover it due to new guidelines. Therefore, you will be paying $200.00 out of pocket for 40 tablets. The pharmaceutical industries are making trillions from the inoculations and countries are bound to their multimillion dollar contracts with Pfizer… why would Pfizer or the Federal/Local Governments support any other treatments?
Gilead was paid $34.5 million to develop Remdesivir by the Department of Defense and is partnered with a pharmaceutical facility in China owned by George Soros.
Currently, hospitals including the Health Sciences of South Carolina, which includes Spartanburg Regional and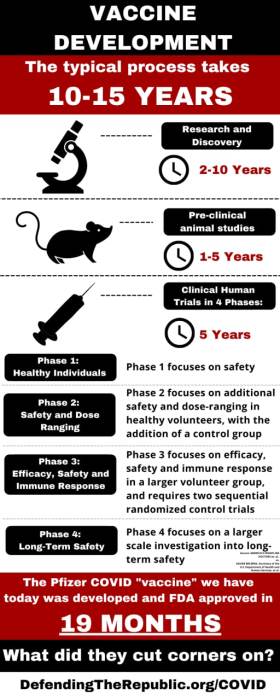
Gilead, who was paid $34.5 million to develop Remdesivir by the Department of Defense, is partnered with a pharmaceutical facility in China owned by George Soros. Once the investigational Remdesivir was approved for EUA use in 2020 despite poor efficacy and dangerous outcomes, the drug was moved to China where researchers from the Wuhan Institute of Virology filed an application to patent Remdesivir. Keep in mind that recently released FOIA documents by The Intercept showed that Fauci initiated funding of gain of function research at the Wuhan Institute of Virology to develop another SAR-CoV-2 virus. Further, Remdesivir is upwards of $2,000 dollars while ivermectin and hydroxychloroquine are a mere $10.
Since the inception of the FAERS database in 1996:
-
- Ivermectin averages 15.2 deaths per year (total of 379 deaths over 25 years of data).
- HCQ averages 48.3 deaths per year (total of 1,641 deaths over 34 years of data).
- Remdesivir resulted in 1,499 deaths in just one year.
Since December 2020, the COVID Vaccines have led to over 14,000 deaths in just 1 of 11 vaccine injury reporting databases. A lawsuit filed against the Department of Health and Human Services in Alabama by Lawyer Thomas Renz and America’s Frontline Doctors provides whistleblower testimony asserting that the Centers for Medicare and Medicaid Services (CMS) databases show that roughly 45,000 elderly and disadvantaged died within 3 days of the inoculation.
According to a study published in April 2020, those hospitalized and placed on Remdesivir treatment for COVID had a risk of death 85.7% higher than the control group. Another study noted a 100% risk of death when using Remdesivir compared to the control group. In another study published in May 2020, Remdesivir was stopped early because of serious adverse events to include respiratory failure or acute respiratory distress syndrome. It has been reported to be largely ineffective for COVID by numerous uncensored news outlets. The New York Times, Forbes and MSN have all reported it ineffective in COVID 19 treatment.
It should be alarming to you that government and hospitals across the country are denying efficacious treatment, inoculating individuals with dangerous drugs, and doing so in the name of “policy”. This policy is proving to be deadly.
Elizabeth Shewfelt has worked in healthcare management conducting pharmaceutical clinical trials and is now affiliated with a national healthcare billing organization. She has direct experience in many facets of the healthcare industry from lab processing human specimens to clinical trial research. She has worked in healthcare for the federal government, forensic psychology firms and in an industrial organizational psychology firm. She has 3 masters degrees associated with healthcare. She is passionate about is education and knowledge and lives in Charleston, SC. We are glad to welcome Elizabeth to our staff.
Please “like”, comment, share with a friend, and donate to support The Standard on this page. Become a Patron!
Click the QR Code below to donate any amount.





 RSS - Posts
RSS - Posts
What a well-documented and researched article. The Standard always delivers on up to the minute, relevant news. Thank you, Michael Reed!
My daughter has worked for Grow Financial for 7 years (the last 4 years at home). She just won an award at work… she already had covid… she didn’t want to get the shot because she was trying to get pregnant and was worried about how it would affect the baby. They told her you either get the shot or you’re fired, so they fired her!